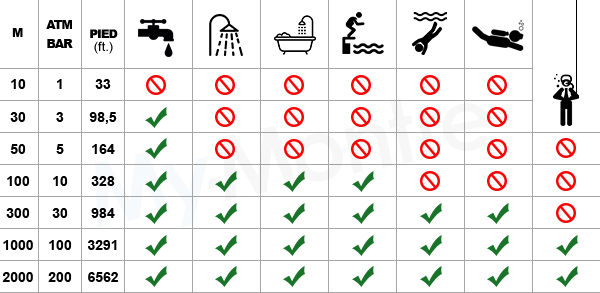
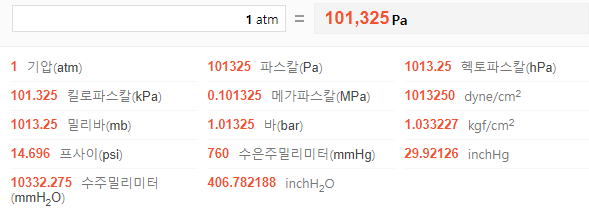
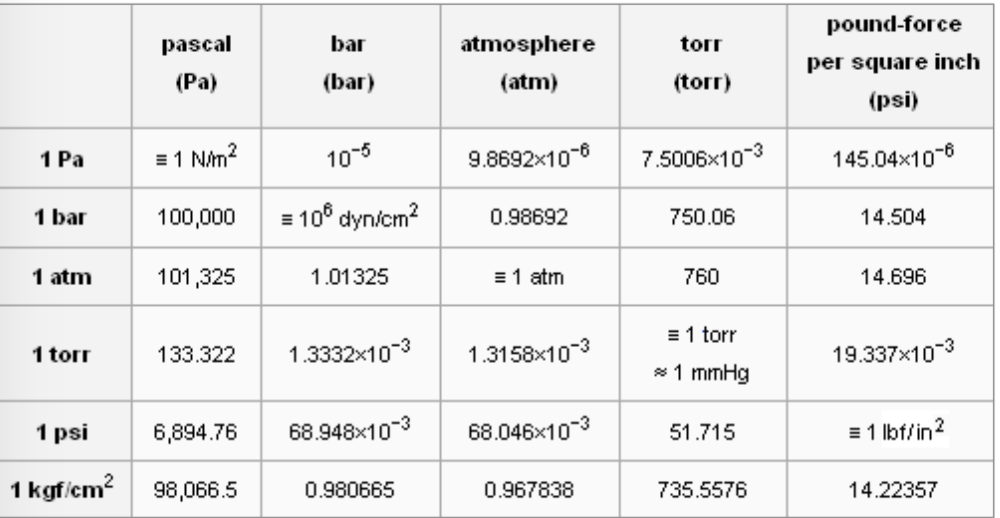
Is this incorrect? Standard Condition = 298K, 1 ATM and 1 M, STP = 273K, 1 ATM, 22.4 L. Shouldn't the card say Standard Condition is 298K? : r/Mcat

Diagramme X-Y pour le mélange réactif à P = 1 atm. X-Y diagram for the... | Download Scientific Diagram
The pressure of hydrogen gas is increased from 1 atm to 100 atm. Keeping the H (1 M) constant, the voltage of the hydrogen half cell at 25^° C will be: (a)

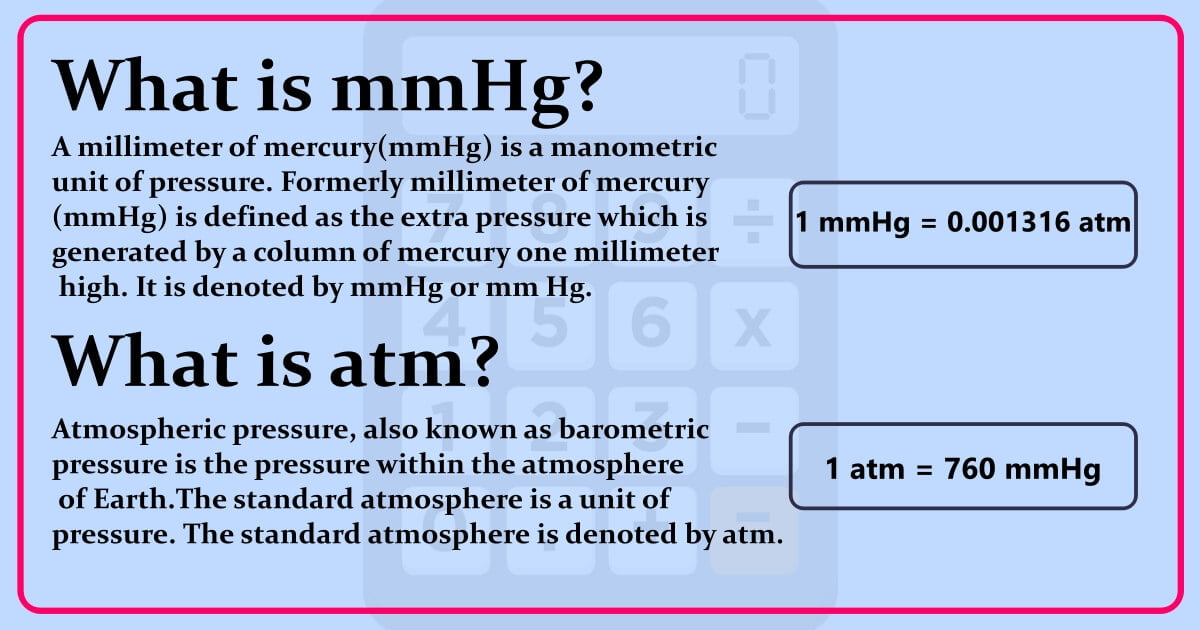
SOLVED: 1 atm = 760 mm Hg 760 torr = 1 atm 101325 Pa = 1 atm 101.3 J = 1 L atm 1 nm = 1 x 10^-9 m 0.08212 L

Gas Pressure Unit Conversions - torr to atm, psi to atm, atm to mm Hg, kpa to mm Hg, psi to torr - YouTube

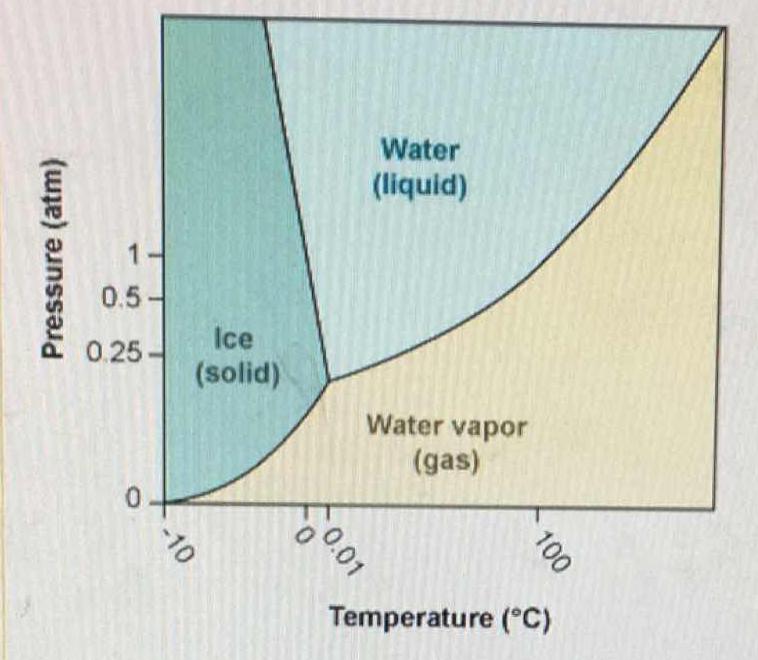
Déterminer l'état physique de l'eau à l'aide de sa température, sa pression et du diagramme d'état de l'eau - Tle - Exercice Enseignement scientifique - Kartable

Volume of 0.5 mole of a gas 1 atm. pressure and 273 K is 22.4 litres11.2 litres5.6 litres44.8 litres
![SOLVED: Conversion Factors 1 gal = 231 in' (exact) 1 atm 760 torr (exact) cm = ] mL (exact) 1 b = 453.59237 g (exact) 2.54 cm = L in (exact) 1 SOLVED: Conversion Factors 1 gal = 231 in' (exact) 1 atm 760 torr (exact) cm = ] mL (exact) 1 b = 453.59237 g (exact) 2.54 cm = L in (exact) 1](https://cdn.numerade.com/ask_images/a1b97c8ec9ce48bcb5b41f914204e5b4.jpg)
SOLVED: Conversion Factors 1 gal = 231 in' (exact) 1 atm 760 torr (exact) cm = ] mL (exact) 1 b = 453.59237 g (exact) 2.54 cm = L in (exact) 1

At 100^oC and 1 atm pressure the density of water vapour is 0.0005970 g/cc.What is the molar volume and how does this compare with ideal gas volume ?What is the compressibilty factor '
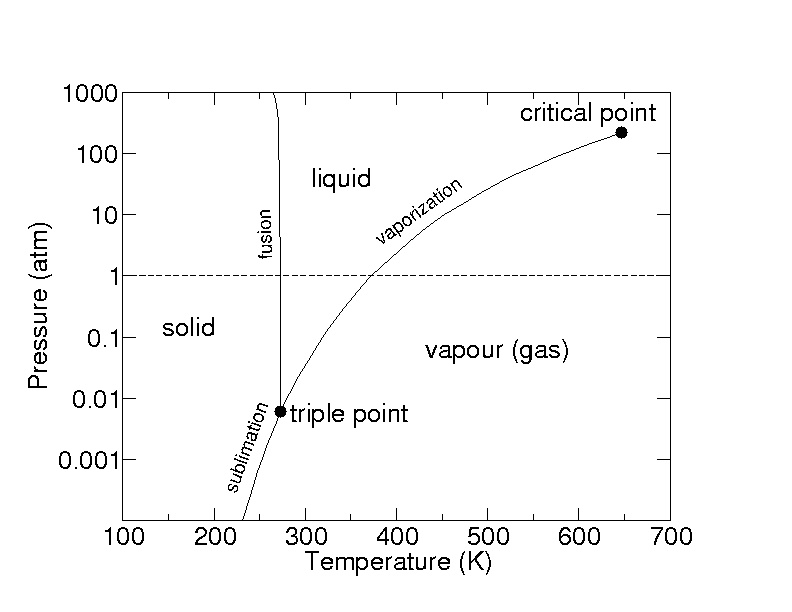
2.5: A pressure-temperature diagram (phase diagram) for water. Water at 1 atm pressure is on the dotted line